Washington, D.C., USA- January 13, 2020: FDA Sign at its headquarters in Washington DC. The Food and Drug Administration (FDA or USFDA) is a federal agency of the USA.
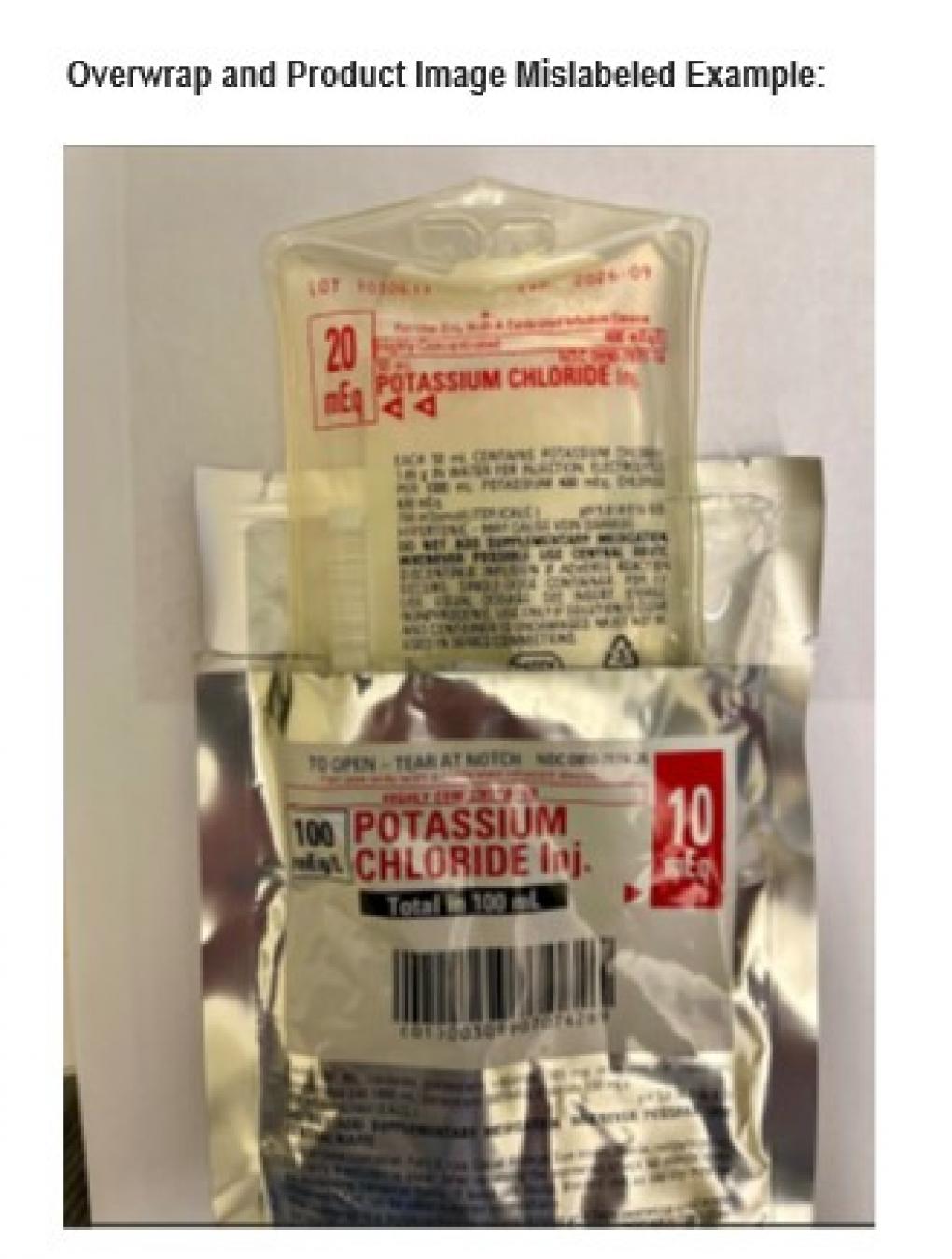
November 4, 2025 — Otsuka ICU Medical has announced a nationwide recall for a specific lot of its 20 mEq Potassium Chloride Injection after it was found that the outer label of the product was incorrectly labeled as a 10 mEq dose. This mislabeling raises concerns about the risk of overdoses if the product is administered incorrectly.
The recall pertains to lot 1030613 (expiration date September 30, 2026) of Potassium Chloride Injection 20 mEq (NDC 0990-7077-14). The labeling error occurred on the product’s overwrap, which incorrectly states the solution as 10 mEq (NDC 0990-7074-26). The accurate dosage is only displayed on the inner product label, which is not visible while the overwrap is still intact.
The company has indicated that the mislabeling is due to a manufacturing error. While there have been no reported adverse events to date, this mistake could result in a double dose of potassium chloride, potentially leading to hyperkalemia—a serious condition characterized by dangerously elevated potassium levels in the bloodstream.
Potential Health Risks, Distribution and Product Details
Excessive intake of potassium chloride can lead to significant muscle weakness, paralysis, confusion, decreased blood pressure, irregular heartbeats, and in severe situations, cardiac arrest and death. Patients who are particularly vulnerable include premature infants, those with kidney issues, individuals taking potassium-sparing diuretics, or patients on long-term parenteral nutrition.
The lot in question was produced on April 15, 2025, and was distributed throughout the United States from May 23 to August 26, 2025. This recall is limited to this particular lot; other potassium chloride products from Otsuka ICU Medical remain unaffected.
Company Response and Guidance
Otsuka ICU Medical has instructed healthcare providers, hospitals, and pharmacies to cease the use and distribution of the recalled product immediately and to return it to the point of purchase. Customers who need assistance or return labels can reach out to the recall support center, Sedgwick, at 1-888-566-2363.
Potassium chloride injections are generally utilized to address potassium deficiency when oral supplementation is not feasible. Given their high concentration, these solutions must be administered gradually and with continuous cardiac monitoring to avoid hazardous spikes in potassium levels.
The company stressed that ensuring patient safety is its highest priority and that it is collaborating with regulators to guarantee the prompt removal of the affected products from the market.
| NDC Number | List Number | Product Description | Lot Number | Expiration Date | Configuration |
|---|---|---|---|---|---|
| 0990-7077-14 | 070770452 | POTASSIUM CHLORIDE Inj. 20 mEq | 1030613 | 30 September 2026 | 50mL in Flexible Container |
| 0990-7074-26 | 070740452 | POTASSIUM CHLORIDE Inj. 10 mEq | N/A | N/A | 100mL in Flexible Container |
DESCRIPTION OF CASES BEING RECALLED:
| NDC Number | Barcode Number | Lot Number | Expiration Date | Configuration |
|---|---|---|---|---|
| 0990-7077-14 | (01)20309907077141 | 1030613 | 30 September 2026 | 24/case |